
Replacing traditional fuel-powered vehicles with battery-powered options is essential to reduce carbon dioxide (CO2) emissions. This greenhouse gas results from the combustion of fossil fuels, therefore limiting its input into the atmosphere will also influence global warming. Battery production for electric vehicles and other rechargeable devices (e.g., cell phones or laptops) requires stringent quality control and testing to ensure the best performance. Meanwhile, battery research focuses on discovering new battery materials with higher energy and power density as well as a more efficient energy storage.
In this blog post, I want to highlight a few of the analytical parameters which can be determined using high precision analytical instruments from Metrohm and provide some free downloads in this research area.
Learn about the following points in this article (click to go directly to each topic):
What’s in a lithium-ion battery?
Today, lithium-ion batteries are the most common rechargeable batteries available on the market. A battery consists of an anode (negative electrode) and a cathode (positive electrode). An electrolyte facilitates charge transfer in the form of lithium ions between these two electrodes. Meanwhile, a separator placed between anode and cathode prevents short-circuits. An example cross-section can be seen in Figure 1.
The anode is made from graphite containing intercalated lithium applied to a copper foil, while the cathode consists of lithium metal oxides applied to an aluminum foil. Cobalt, nickel, manganese, or iron are the most commonly used transition metals in cathode materials. The electrolyte is an anhydrous aprotic solvent containing a lithium salt (e.g., lithium hexafluorophosphate) to facilitate charge transfer. The separator is an isolator made from a porous material, allowing the migration of lithium ions for charge transfer. The composition of all of these components has a significant influence on the battery characteristics.
After this brief overview about the composition of a lithium-ion battery, let’s take a look at selected key parameters and how they can be analyzed.

Water content in battery raw materials
Lithium-ion batteries should be free of water (concentration of H2O less than 20 mg/kg), because water reacts with the conducting salt (e.g., LiPF6) to form toxic hydrofluoric acid. Sensitive coulometric Karl Fischer titration is the ideal method for determining water content at trace levels. Water determination for solids is carried out using the Karl Fischer oven method – the residual moisture in the sample is evaporated and transferred to the titration cell where it is subsequently titrated.
Automated sample preparation for Karl Fischer Titrandos
The working principle and advantages of the KF oven method are described in more detail in our blog post below.
Oven method for sample preparation in Karl Fischer titration

For more details on how to carry out the water determination in one of the following battery components, download our free Application Bulletin below:
- raw materials for the manufacture of lithium-ion batteries
- electrode coating preparations (slurry) for anode and cathode coating
- the coated anode and cathode foils as well as in separator foils and in packed foil layers
- electrolytes for lithium-ion batteries

Transition metal composition of cathode materials
The cathode of a lithium-ion battery is usually made from metal oxides derived from cobalt, nickel, manganese, iron, or aluminum. To produce the cathode, solutions containing the desired metal salts are used. For an optimized production process, the exact content of the metals present in the solution must be known. Additionally, the metal composition within the obtained cathode material should be determined. Potentiometric titration is a suitable technique to determine the metal content in starting solutions and the finished cathode materials.
Unlike competing methods such as ICP-MS or AAS, titration does not require dilution of such samples. Hence, results obtained by titration are more reliable and accurate. Furthermore, running and maintenance costs are considerably lower compared with ICP-MS or AAS.
The following mixtures of metals or metal oxides can be analyzed potentiometrically:
- nickel, cobalt, and manganese in solutions
- nickel, cobalt, and manganese in cathode materials such as lithium nickel manganese cobalt oxide (NCM), lithium cobalt oxide (LCO), or lithium manganese oxide (LMO)
For more details about the potentiometric analysis of a mixture of nickel, cobalt, and manganese, download our free Application Note below.
Analysis of Li-ion battery cathode materials made from Co, Ni, and Mn

Analysis of lithium salts
Potentiometric titration is also ideally suited for determining the purity of lithium salts. For lithium hydroxide (LiOH) and lithium carbonate (Li2CO3), the purity is determined using an aqueous acid-base titration. It is also possible to determine carbonate impurity within LiOH using this method.
For more details about performing the assay of LiOH and Li2CO3, download our free Application Note here.
For the assay of lithium chloride (LiCl) and lithium nitrate (LiNO3), the lithium is directly titrated using the precipitation reaction between lithium and fluoride in ethanolic solutions. For more details about how to carry out the assays of LiCl and LiNO3, download the following free Application Notes.
Lithium in brine – Reliable and inexpensive determination by potentiometric titration
Assay of lithium nitrate – Reliable and fully automated determination by potentiometric titration
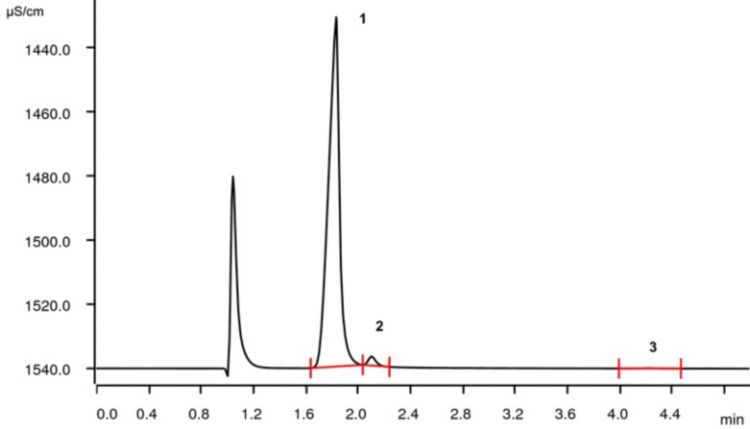
The knowledge of other cations which might be present in lithium salts (and their concentration) is also of interest. Various cations (e.g., sodium, ammonium, or calcium) can be determined using ion chromatography (IC). IC is an efficient and precise multi-parameter method to quantify anions and cations over a wide concentration range.
The chromatogram in Figure 2 shows the separation of lithium, sodium, and calcium in a lithium ore processing stream.
For more information on how this analysis was carried out, download our free Application Note here.

Electrolyte composition
The lithium ion is responsible for charge transfer within lithium-ion batteries. Lithium hexafluorophosphate (LiPF6) is the main conductive salt. However, LiPF6 tends to decompose at elevated temperatures, or it can react with traces of water to form toxic hydrofluoric acid. Therefore, lithium borate salts or imide-based lithium salts are used as additives to improve its performance. Ion chromatography (IC) allows the determination of decomposition of the different lithium salts within the electrolyte. Additionally, IC can be used to analyze ionic impurities at trace levels. Furthermore, any sample preparation steps that might be required (e.g., preconcentration, dilution, filtration) can be automated with the Metrohm Inline Sample Preparation («MISP») techniques.
For more detailed information about selected IC applications for battery research, check out our Application Notes:
Summary
This blog post contains only part of the analyses for battery research which are possible using Metrohm’s analytical instruments. Part 2 discusses the electrochemical characterization of batteries and their raw materials.
 Udostępnij artykuł
Udostępnij artykuł
