In the past, lead was commonly used as a stabilizer in electroless nickel plating processes. The regular and precise determination of the stabilizer concentration is essential to keep the plating process under stable conditions and to run the process successfully. As restrictions on the use of lead in consumer products (particularly electronics) have grown in recent years, alternative stabilizers were developed and introduced. One of the stabilizers used as a lead replacement is iodate (IO3- ).
Electroless nickel plating is used in various industrial production processes (e.g., production of hard disks, and for protection against corrosion or wear). The ENIG (electroless nickel, immersion gold) and ENEPIG (electroless nickel, electroless palladium, immersion gold) processes in the production of printed circuit boards (PCB) are very reliant on the success of this method as electroless nickel plating is the first step in the process.
Polarography can be used to determine the iodate content after dilution in supporting electrolyte and has been established as a straightforward, sensitive, selective, and interference-free method for this application.
Electroless nickel plating bath
After diluting the sample in electrolyte, the polarographic determination of iodate is carried out on the 884 Professional VA with the Multi-Mode Electrode pro as working electrode using the parameters listed in Table 1. The concentration of iodate is determined by two additions of standard addition solution.
| Parameter | Setting |
|---|---|
| Working electrode | DME |
| Mode | DP – Differential Pulse |
| Start potential | -0.1 V |
| End potential | -0.7 V |
| Peak potential iodate | -0.34 V |
- Working electrode: Multi-Mode Electrode pro with standard glass capillaries
- Reference electrode: Ag/AgCl/KCl (3 mol/L) reference electrode with electrolyte vessel. Bridge electrolyte: KCl (3 mol/L)
- Auxiliary electrode: Platinum rod electrode
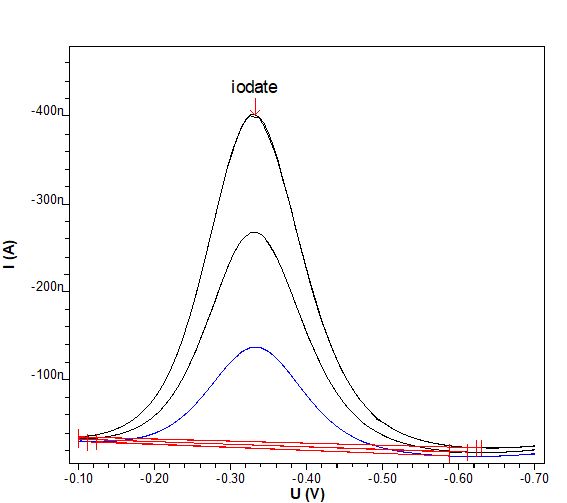
The determination of iodate in electroless nickel plating baths can be carried out in a simple and straightforward manner. The method is selective and free of interferences. It is suitable for concentrations in the low mg/L range.
| Sample | Concentration IO3- [mg/L] |
|---|---|
| Electroless nickel bath | 2.5 |
Internal reference: AW CH4-0475-092007
 Share via email
Share via email
 Download PDF
Download PDF