Mercury and its compounds are toxic. The highest risk is posed by chronic poisoning with mercury compounds ingested with food. A significant part of the mercury present in the environment is of anthropogenic origin. Considerable sources are coal-fired power plants, steel, and nonferrous metal production, waste incineration plants, the chemical industry, or artisanal gold mining where the use of elemental mercury for the extraction of gold from the ore is still common. The guideline value for inorganic mercury in the World Health Organization’s «Guidelines Quality» for Drinking-water is set to 6 μg/L.
With a limit of detection (LOD) of 0.5 μg/L, anodic stripping voltammetry is a viable, less sophisticated alternative to atomic absorption spectroscopy (AAS). While AAS (and competing methods) can only be performed in a laboratory, anodic stripping voltammetry can be used conventionally in the laboratory or alternatively in the field with the 946 Portable VA Analyzer. The determination is carried out on the scTRACE Gold electrode.
Bottled mineral water, spiked
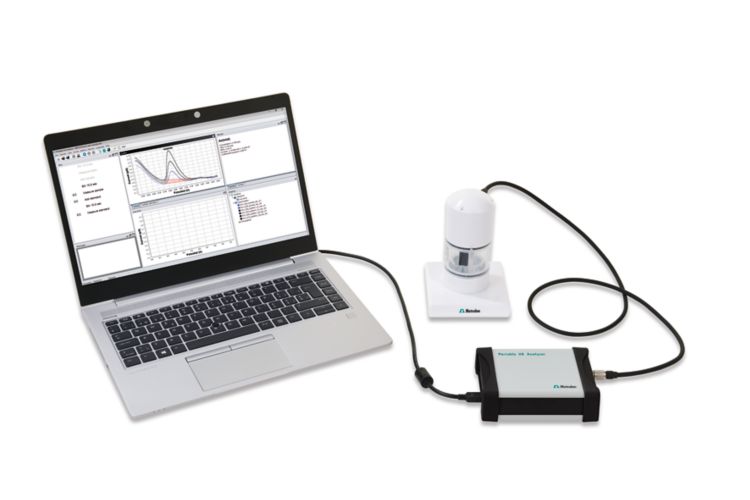
The scTRACE Gold is electrochemically activated and an ex situ mercury film is deposited prior to the first determination. In the next step, the water sample and the supporting electrolyte are pipetted into the measuring vessel. The determination is carried out with the 884 Professional VA or with the 946 Portable VA Analyzer using the parameters specified in Table 1. The concentration is determined by two additions of a standard addition solution.
| Parameter | Setting |
|---|---|
| Mode | SQW – Square wave |
| Deposition potential | 0.3 V |
| Deposition time | 90 s |
| Start potential | 0.3 V |
| End potential | 0.6 V |
| Peak potential As | 0.44V |
- scTRACE Gold
The limit of detection of the method is approximately 0.5 μg/L.
| Sample | Hg (μg/L) |
|---|---|
| Bottled mineral water | 2.1 |
Application Bulletin 422: Determination of mercury in water with the scTRACE Gold
 Share via email
Share via email
 Download PDF
Download PDF