Anyone requiring general, scientific, or industrial material analysis, including:
- Defense/Security professionals
- Chemists
- Forensics analysts
- Workers at receiving docks
- People working in research and education
As the spectrometer’s laser interacts with a sample, the energy of the light scattered back is shifted resulting in a Raman spectrum that gives valuable information about the chemical structure. This article covers some of the most frequently asked questions about Raman spectroscopy regarding the theory behind it and how it can be used in practice.
Click on a question below to jump directly to that subject:
Raman is a form of molecular spectroscopy that is observed as inelastically scattered light when a sample is excited by a laser. While most scattering occurs elastically, about 1 in 106 scattering processes interact with the molecule through bond stretching and bending vibrations resulting in Raman-scattered light. Shifted by these molecular interactions, the detected Raman photons can be processed into a spectrum that relates to the unique bonds within a molecule, providing the user with an invaluable analytical tool for molecular fingerprinting. This «fingerprint» is used primarily for material identification and, increasingly, for quantification.
Note: molecular vibrational spectroscopies only detect two or more atoms that have a molecular bond between them—salts, ions, and metals require other analytical methods.
Raman spectroscopy can be used to identify most materials that are present at a sufficient quantity and purity and/or in simple mixtures. Raman can identify thousands of solid and liquid substances including pharmaceuticals, raw materials for food and personal care products, controlled substances and associated precursors and cutting agents, weapons of terror, toxic and non-toxic chemicals, solvents, and agricultural treatments (e.g., pesticides, insecticides).
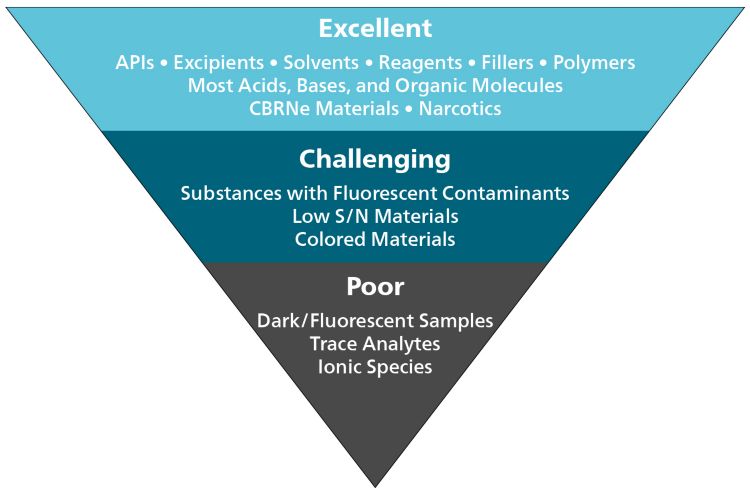
Following are some general guidelines:
Fluorescence is traditionally the biggest limitation for Raman. It is a much more efficient emission process, causing overwhelming background noise in the Raman spectrum and obscuring Raman peaks. Natural substances (such as plant fibers), strongly colored materials, and substances with fluorescent contaminants can all fail to produce results with Raman spectroscopy. Luckily, this limitation is not insurmountable.
A common solution has been to move the wavelength of the excitation laser away from the absorbance wavelength of the material – typically 532, 638, or 785 nm. The most common choice of wavelengths for reduced fluorescence effects is 1064 nm.
How do you know which wavelength is most suitable? Read our free Application Note for some tips.
Application Note: Choosing the Most Suitable Laser Wavelength For Your Raman Application
Metrohm Raman uses its own proprietary method in MIRA XTR DS, a proven handheld 785 nm Raman system equipped with fluorescence rejection. Find out more about this unique solution in our White Paper.
White Paper: Fluorescence-free 785 nm material ID with MIRA XTR DS
Peaks in the Raman spectrum are very narrow, which heightens specificity and selectivity. Therefore, it can differentiate very similar materials or identify target analytes in mixtures. Raman is great for structural elucidation of molecules, including connectivity and saturation. Unique fingerprint peaks in the Raman spectrum can be used to discriminate between very similar species such as isomers and substances that differ by a single functional group.
Raman spectroscopy can help users observe the progression of a chemical reaction, differences in crystallinity between polymorphs, and changes in bond energies that arise from applied stress on a material. The following Application Note offers even more insight into this kind of study.
Intensity in the Raman spectrum is directly proportional to sample concentration and can also be used for quantitative analysis. Learn more in our free Application Note below.
Application Note: Quantitative Analysis of a Water-soluble Polymer Using the i-Raman EX Spectrometer
Although the Raman spectrum has a potential range from 0–4000 cm- 1, most applications can be satisfied with a narrower spectral range. The fingerprint region, 400–1800 cm-1, largely reveals the molecular environment of atoms. This is adequate for identification of unknowns and verification of materials (see image below), both of which rely on the identity of the molecular structure.
Outside of the fingerprint region, simple carbon chains and hydrogen attachment contribute little to material identification. However, the high wavenumber region is actively being researched in the medical field for cancer research, human dental issues, and biofuels. Niche applications such as crystal structure in minerals, gemology, organometallics, and semiconductors require information below 400 cm-1.
Raman is a powerful analytical technique:
To summarize, the appeal of Raman spectroscopy is its broad applicability by non-technicians in non-traditional settings. Raman takes analytical chemical capabilities out of the lab and provides instant material identification right where it’s needed: at the receiving dock, in food production facilities, museums, clandestine labs, for process analytics, or even at the border. All of these are ideal scenarios that benefit from the strengths of Raman.
Our Real World Raman series demonstrates the benefits of handheld Raman in non-technical settings.
Real World Raman: Simplifying Incoming Raw Material Inspection
Real World Raman: MIRA DS in Action
Real World Raman: Exposing fentanyl-laced, counterfeit, and illegal drugs
Identification of unknowns is a measure of spectral similarity between the unknown substance and library spectra. This method of identification is easy to implement, fast, and suitable for use with extensive, customizable chemical libraries. An example of this technique would be on-site testing of a small bag of white powder confiscated at a traffic stop, providing fast evidence of illegality at the point of contact without exposing the authorities to any potential hazards. Download our White Paper below for more information on this subject.
White Paper: Identifying Narcotics in Complex Samples
Raman’s selectivity also makes it an excellent technique for verification of known materials, which confirms the consistency, purity, and quality of raw materials for manufacturers of food, pharmaceuticals, hair and skincare products, cosmetics, and more. The verification method detects slight spectral differences by projecting each sample spectrum onto a model. This either passes or fails based on how well the sample spectrum fits the model. Find out more about verification with Raman spectroscopy in the following White Paper.
White Paper: Verification, p-values, and Training Sets for the Mira P
Anyone requiring general, scientific, or industrial material analysis, including:
In the laboratory, at manufacturing facilities, crime scenes, or at the border.
Portable and handheld systems are able to travel with the user directly to the testing location.
When the identification, verification, or distinction of sufficiently pure substances is desired—especially when dealing with unknown white powders and synthetic materials.
Guided and automated workflows reduce sampling to a three or four step procedure, giving results in seconds without any hassle.
To determine the consistency of ingredients, find out whether something is hazardous, identification of a suspicious substance, or confirming the identity of a material.
Surface-enhanced Raman scattering (SERS) is a specialized Raman technique that helps users detect trace amounts of substances. Not all materials are SERS-active, but strongly SERS-active materials can be detected at parts-per-million (ppm, mg/L) or parts-per-billion (ppb, µg/L) levels. SERS can also be used to detect a specific component in a mixture or identify strongly colored dyes and materials, as it is not susceptible to fluorescence.
The biggest challenge for SERS is the detection of a target compound in complex matrices, including in water, pills (e.g., either regulated pharmaceuticals, over the counter drugs, or those sold on the street), and a variety of foodstuffs. With experience and investigation, the unique properties of SERS analysis can be exploited with simple sample preparation.
Find out more about how SERS compares to Raman in our previous blog post.
Ultimately, Raman spectroscopy is an ideal technique for material identification or verification that is available to technical and non-technical users in a wide variety of settings. Raman is easily implemented, preserves the sample, and can be used to analyze thousands of materials. To learn more about Raman and its many benefits, check out our other blog articles, our Application Notes, and White Papers.