Arsenic is ubiquitous in the earth’s crust in low concentrations. Elevated levels can be found in mineral deposits and ores. Arsenic from such deposits leaches into the groundwater in the form of arsenite (AsO33-) and arsenate (AsO43-), causing its contamination. As(III) is more toxic than As(V) and shows higher mobility in the environment. The selective determination of this species is possible using the method described in this document.
With a limit of detection (LOD) of 0.3 μg/L, anodic stripping voltammetry allows speciation, i.e. the specific determination of As(III). While atomic absorption spectroscopy (AAS) (and competing methods) can only determine the total element concentration, anodic stripping voltammetry is selective to the As(III) oxidation state. The determination is carried out on the scTRACE Gold electrode.
Bottled mineral water
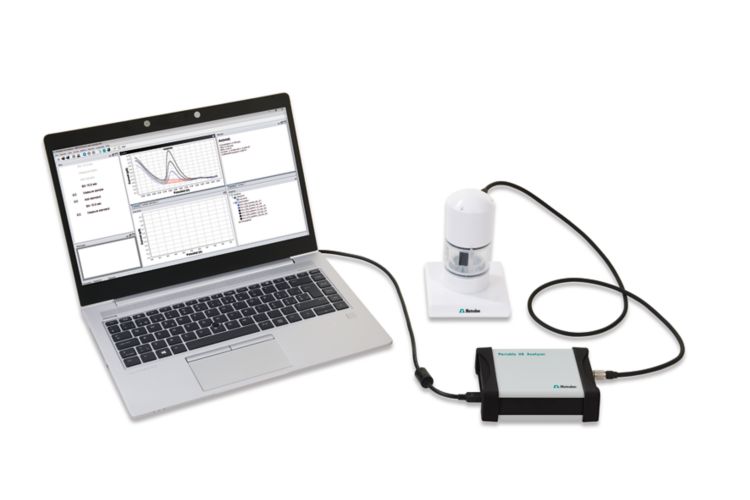
The scTRACE Gold is electrochemically activated prior to the first determination. In the next step, the water sample and the supporting electrolyte are pipetted into the measuring vessel. The determination of arsenic is carried out with the 884 Professional VA or with the 946 Portable VA Analyzer using the parameters specified in Table 1. The concentration is determined by two additions of an arsenic standard addition solution.
| Parameter | Setting |
|---|---|
| Mode | SQW – Square wave |
| Deposition potential | -0.5 V |
| Deposition time | 60 s |
| Start potential | -0.3 V |
| End potential | 0.4 V |
| Peak potential As | 0V |
- scTRACE Gold
With a 60 s deposition time, this method is suitable for the determination of arsenic in water samples in concentrations from β(As(III)) = 0.3–10 μg/L.
| Sample | As (μg/L) |
|---|---|
| Bottled mineral water | 1.4 |
Application Bulletin 416: Determination of arsenic in water with the scTRACE Gold
 Share via email
Share via email
 Download PDF
Download PDF