Titrants are normally bought ready to use. However, the certified value is only valid at a defined temperature because the titrant density varies with temperature. With time and especially after opening the bottle of your titrant, the properties thereof will change because of evaporation of water and uptake of carbon dioxide. Due to these facts, it is necessary to determine the accurate concentration of your titrant solution on a regular basis using a primary standard. To correct the mentioned variation, a socalled «titer factor» is applied.
In case of hydrochloric acid as titrant, the primary standard to use is TRIS (Tris(hydroxymethyl)aminomethan). TRIS is inert, does not sublimate, and reacts with hydrochloric acid according to a defined chemical reaction.
The titer can be easily and quickly assessed by using the Metrohm brand of autotitrators. Predefined calculation formulas implemented in Metrohm titrators or software, respectively, as well as the automatic storage of the titer factor, makes standardization a simple task.
High purity TRIS is used for the standardization of hydrochloric acid. TRIS is dried in a drying oven for several hours and allowed to cool down to ambient temperature in a desiccator.

An appropriate amount of TRIS standard is added accurately to a beaker and dissolved with deionized water. The solution is titrated against hydrochloric acid until after the equivalence point is reached.
The sample size must be chosen according to the buret volume (equivalence point between 10–90% of buret volume).
If a small cylinder unit (2 or 5 mL cylinder unit) is used for titration, it is recommended to make a stock solution and use an aliquot thereof for titration. This increases the accuracy for these burets.
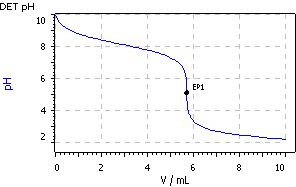
A six-fold determination exhibited a mean titer value of 1.0069 with an absolute standard deviation of 0.0037 and a relative standard deviation of 0.37%.
The determination of the titer of hydrochloric acid is performed both quickly and reproducibly.
Easy, fast, and precise titer determination using Metrohm autotitrators results in reliable titration analyses. Predefined calculation formulas implemented in these titrators or software, respectively, as well as the automatic storage of the titer factor makes standardization a simple task.
 Share via email
Share via email
 Download PDF
Download PDF