Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis.
Founded in 1943 by Bertold Suhner in Herisau, Switzerland, Metrohm has grown to an international company that supplies its products in more than 80 countries through more than 37 own subsidiaries and numerous exclusive distributors.
1 downloads
Staying true to who we are – Our values

Metrohm is still firmly rooted where it was founded: in Herisau, Switzerland.
This is where most of our analytical instruments are engineered, designed, developed, and produced. We believe in doing things right: That’s why we produce as much as possible ourselves, from the hardware to the software, the printed circuit boards and finally the sensors, separation columns, and accessories. This allows us to ensure consistent quality across our portfolio and the different components of our instruments.
Our mission
- Understanding our customers and their needs.
- Thinking globally, acting locally.
- Delivering pioneering products and services for professional and sustainable solutions.
- Focusing on intensive and innovative in-house development.
- Maintaining our financial independence.

True to the spirit of our founder, Bertold Suhner, we have maintained our independence, allowing us to do things the Metrohm way. Metrohm is fully owned by the Metrohm Foundation, which was created by the company founder in 1982.
Since then, we have come a long way: We provide our products and services around the globe, through a network of subsidiaries, exclusive distributors, and regional support centers. While we have grown and changed over the years, we have stayed true to Mr. Suhner's values.
Our vision and values
Metrohm analysis instruments and methods allow our customers to work in a more accurate, reliable, environmentally compatible, and cost-effective way – helping them to be more successful than their competitors.
- Modesty
- Integrity
- Perfection
- Passion
- Curiosity
- Perseverance
- Trust
- Fairness
2 downloads
Sustainability, compliance, quality – Our commitment

We excel in analytical chemistry for a better world!
We have made it our mission to deliver pioneering and sustainable solutions to our customers.
As a globally active enterprise, we are aware of our economic, social, and environmental responsibilities – and we act accordingly.
- We have been awarded Silver Recognition Level by EcoVadis, a group that assesses the quality of companies as suppliers, placing us in the top 30% of companies with regard to sustainability and CSR practices.
- Our strong commitment to environmental transparency is confirmed by CDP, a not-for-profit charity co-funded by the European Union that runs a global disclosure system for investors, companies, cities, states, and regions.

Quality you can trust.
We at Metrohm value quality – it has been in our DNA since 1943.
From application support to training, we've got you covered.
- Expert consulting and application support
- Installation, repair service, and preventive maintenance
- Warranties and spare part availability
10 downloads
Get to know our products
Comprehensive solutions for laboratory analysis

We are the global market leader in analytical instruments for titration.
But we cover many other methods for chemical analysis as well: instruments for ion chromatography (IC), near-infrared spectroscopy, pH and conductivity measurement, voltammetry and CVS, oxidation and stability testing, and liquid handling (LQH).
Online process analysis: Metrohm Process Analytics

Taking our expertise in chemical analysis from the laboratory to industrial production sites, our colleagues at Metrohm Process Analytics, develop a wide range of rugged process analyzers for online process and industrial monitoring in a variety of industries.
Electrochemistry for research and development

Metrohm Autolab, based in Utrecht, the Netherlands, and Metrohm DropSens, based in Asturias, Spain, develop and produce a comprehensive portfolio of instrumentation for electrochemistry, from high-quality potentiostat/galvanostats to customized screen-printed electrodes and accessories.
Handheld and portable Raman spectroscopy analyzers

We are able to provide a diversified portfolio of Raman spectrometers for material verification and identification.
These instruments are engineered and produced by Metrohm Spectro (Plainsboro, NJ) in the USA.
Our Raman instruments can be used for the identification of thousands of substances, such as illicit substances, hazardous materials, and drugs, as well as for the identification of raw materials in the production process.
Application development and know-how – Our expertise

We have always kept a focus on the practical application of our instruments.
If you are facing a particular analytical challenge, you are likely to find a solution among our thousands of applications documented in our databases. If you cannot find it there, we are happy to develop it for you.

Working towards a common goal – our involvement in standard-giving organizations.
Standards influence our daily lives, and their omnipresence has an impact not only on our everyday life but also the economy.
As a leading supplier of equipment for analytical analyses, we cooperate with national and international standardization bodies, such as the United States Pharmacopeia (USP), the American Society for Testing and Materials (ASTM), the Association of Official Analytical Collaboration (AOAC) International, the German Institute for Standardization (DIN), the International Organization for Standardization (ISO), the British Standards Institution (BSI), the French Standardization Association (AFNOR), the Bureau of Indian Standards (BIS), the Standardization Administration of China (SAC), the Swiss Association for Standardization (SNV), and others.
Our cooperation with these organizations aims at defining, improving, and modernizing analytical testing procedures. These efforts, in turn, play an instrumental role in fulfilling safety, quality, and sustainability requirements and affect the safety and quality of life of the general population.
We are here when you need us – Our global presence
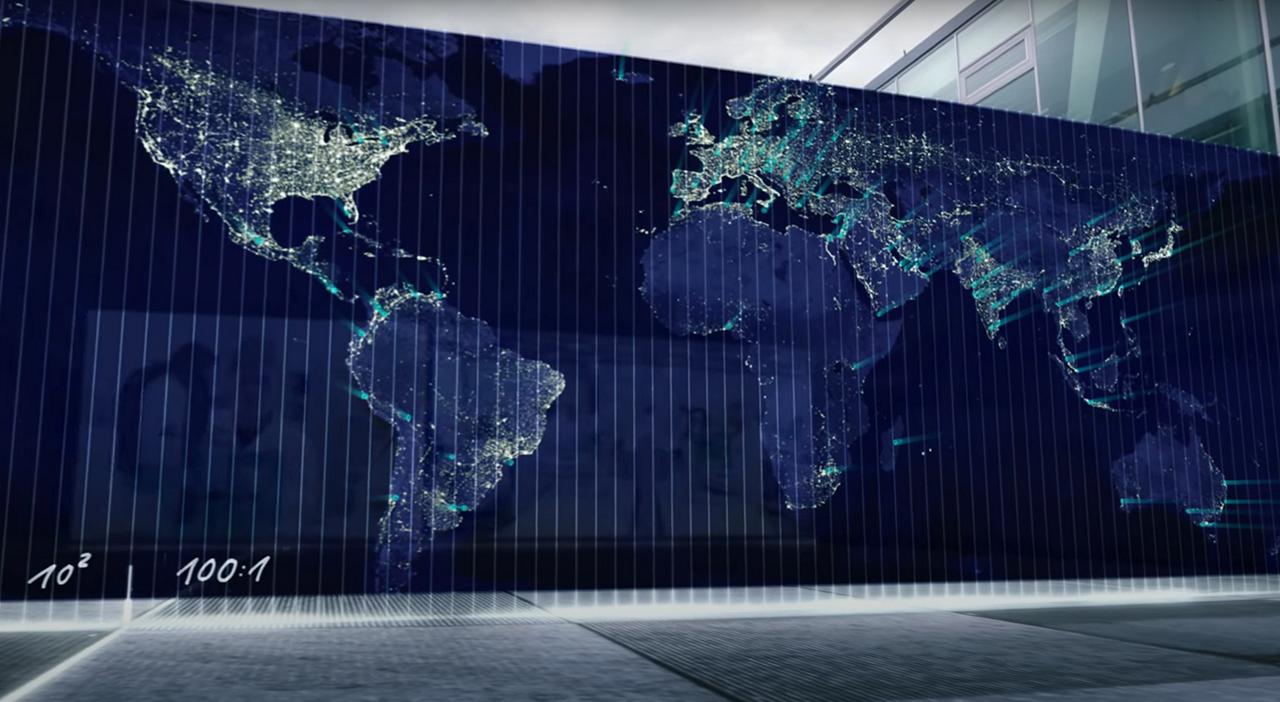
Thanks to our global presence in 80 countries through Metrohm subsidiaries, regional support centers, and a network of exclusive distributors, we are able to ensure fast and local support across the globe.
This allows you to get instrument consulting, application expertise, technical support, and instrument service and maintenance no matter where you are.